We often visualize atoms to look like little solar systems, with the electrons traveling in tidy orbits only a few nuclei widths from the nucleus. In reality, the electrons zip about in cloud-shaped quantum orbitals surrounding the nucleus. In the case of hydrogen, the diameter of the nucleus is about 1.8 x 10-15 meters (1.8 femtometers) while the diameter of the atom with its electron cloud is about 53 x 10-12 meters (53 picometers), nearly 30,000 times larger than the nucleus!
There are very powerful digital 3-d modeling programs for visualizing molecules, but they still cannot provide the same experience as handling and manipulating a physical model. Children truly enjoy assembling models of molecules, so it is worth the investment of time and money to purchase and/or borrow them. These models are great for activities such as our Amino Acids module.
There are a number of different types of molecular models, each with its own strengths and weaknesses for use with younger students. This post is not an exhaustive list of all models available, but those that we have personal experience with and provides a good sample of what’s out there.
Disclaimer: We have no financial ties and receive no financial support or free/discounted supplies from any company mentioned in this post. All recommendations come from our personal experience. We make no money from any links we include. Our Molymod, Ward’s, and Happy Atoms sets were funded by education grants.
Framework Models
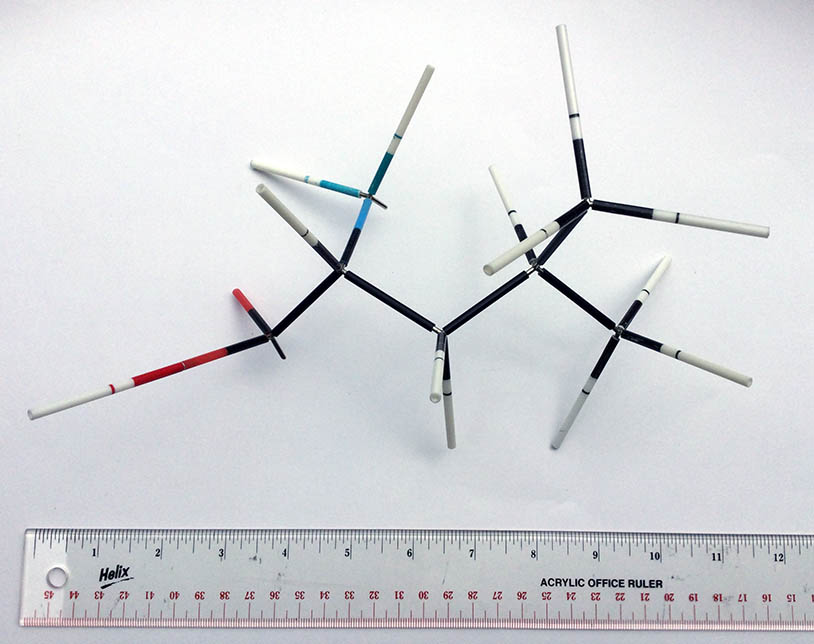
Framework models ignore the electrons and focus on the nuclei. These Prentis Hall models were the type used by college students in the 1970s. They’re apparently not made anymore, but can still be found online. The models consist of hollow plastic tubes (coffee stirrer size) that fit over the spikes of unions that resemble toy jacks. They accurately represent the distance between nuclei and bond angles, and the bonds can rotate like they do in the real molecule.
Framework models like these are still a favorite with chemists who investigate the spatial arrangements of atoms in molecules and the effects of these arrangements on the chemical and physical properties of those molecules. The disadvantages of these models for young students is that it’s hard for them to visualize the intersections of the straws as atomic nucleii, and the tubes can be bent and ruined if forced.
Modern versions of framework moderns are available from various vendors online, with plastic unions/jacks with little integrated spheres representing the atoms. They appear to be more durable and the little spheres should aid with visualization, but we’ve not yet had a chance to try them ourselves. We have ordered a kit and will update this post with our findings.
Ball and Stick Models
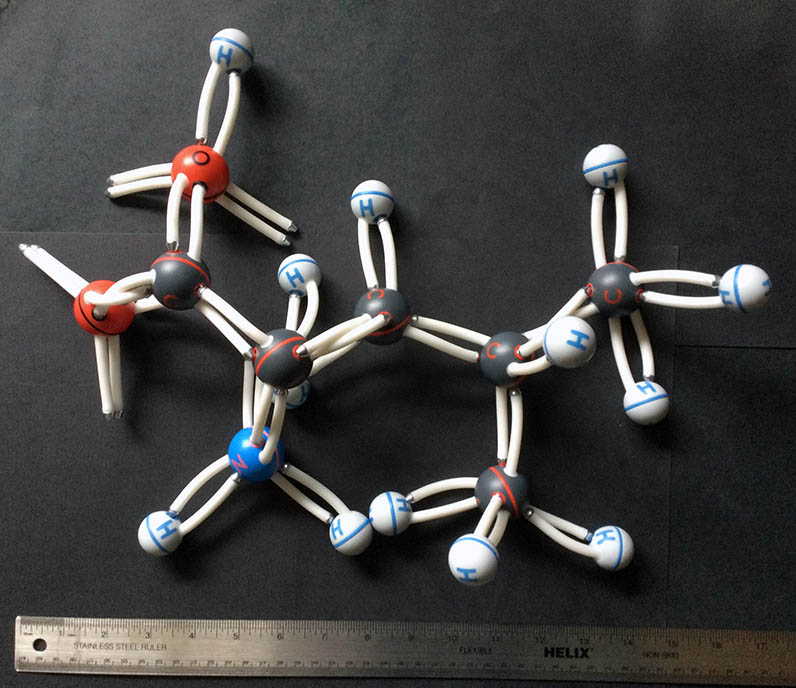
Ball and stick models represent the nuclei with colored balls so it’s easier for young students to visualize the atoms. The balls are connected by one length of bond so the distances between nuclei aren’t accurate.
Molymod
Molymod models use rigid and flexible plastic bonds, easy for children to assemble and take apart, and the models once assembled can be handled without falling apart. The rigid Molymod bonds rotate, and the flexible bonds allow for easy double bond formation. Molymod sets are widely available.
Ward’s Science Spring-Bond
Ward’s Science offers models that use springs for bonds. The students like the springy motion of these models, although they tend to come apart when handled too enthusiastically. The springs are easily damaged if not carefully inserted and removed in a clockwise direction, so they aren’t suitable for younger children. These bonds cannot rotate. Though less-common now, these sets are still sold by Ward’s Science.
Homemade Kits
Our homemade ball and stick set was constructed of wooden beads from the craft store and pieces of chenille (pipe cleaner) stems. The components don’t stay together very well so a crude two-dimensional model is all students could manage. To create a better set, we’d have needed to purchase larger, undrilled wooden balls. This would be more expensive than purchasing commercial sets! This homemade set was retired as soon as we acquired adequate supplies of commercial sets. There are many cases where we recommend homemade options, but this is not one of them. Borrowing or purchasing a commercial model set is well worth it, in our eyes.
Space-Filling Models
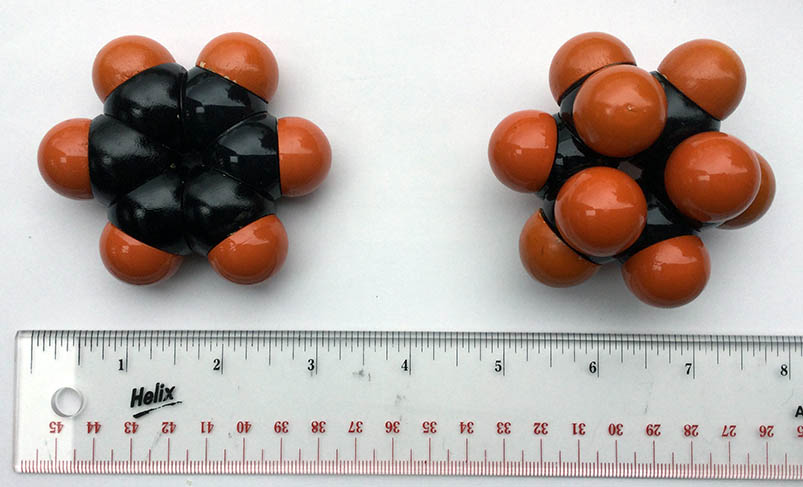
Space filling models take into account the electron clouds, representing the space taken up by the atoms. Bond angles and lengths, and spatial arrangement are accurately represented. Atoms are tightly packed together in space filling models so they are harder for the children to assemble. Students enjoy rotating the bonds of these large and sturdy models, so adult-built models for children to handle and study are useful.
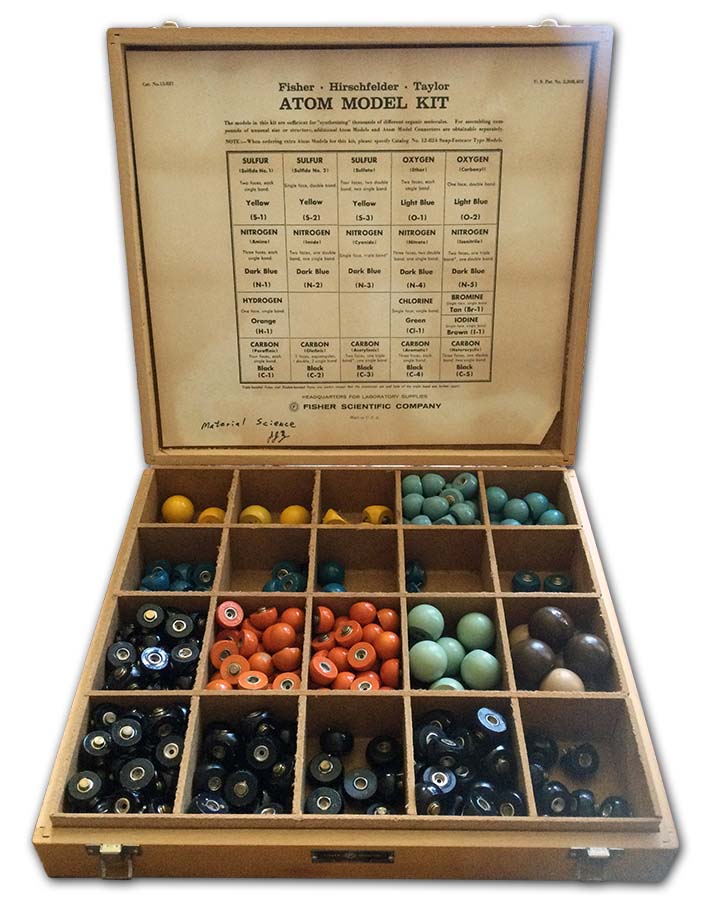
Fisher-Hirschfelder-Taylor
Classic Fisher-Hirschfelder-Taylor models were produced before 1940. Only research labs and universities could afford these very expensive, accurate tools, now consigned to museums or forgotten in storage rooms; our set was rescued on its way to the dumpster. One modern, and likewise very expensive, equivalent is CPK models from Harvard Apparatus. Both sets contain different versions of atoms to create accurate representations of aromatic rings, double and triple bonds, and heterocyclic molecules.
Molymod
Molymod produces components to permit the building of space filling models, including short connectors and versions of atoms required for each type of bond not included in standard student sets. These can be purchased from Indigo Instruments . This style of connector tends to be more difficult for younger children to work with, so we suggest using the ball-and-stick style with these groups.
Other
Happy Atoms
Happy Atoms by Thames and Kosmos is a recent addition to the world of student molecular models. Neither structural or space filling, these models are excellent for demonstrating how atoms share electrons to fill holes in their outer electron shells. These are very easy to assemble with magnetic tipped unpaired “electrons” attached to magnetic “holes” on the surface of the balls representing the atom. The models are large and rather floppy. Happy Atoms comes with a free app. Students can take pictures of their finished models which the app recognizes, responding with the molecule’s name, use, and interesting facts.
Happy Atoms are best used for small, 3-6 atom, models. The floppiness means that molecules much larger than this can be unwieldy to work with, nor do they convey the spatial structure. Happy Atoms also only come in certain sets, so you can end up with extra atom types you don’t need and you can’t buy extras easily and cheaply. These are perhaps our students’ favorite — the app is a particular hit — but are also the most expensive of the currently available student model sets.
Verdict
We recommend using Molymod-style ball and stick models with elementary school students. These are the easiest for students to assemble, and difficult to damage. A cost- effective way to obtain additional atoms — one never has enough carbon and hydrogen — can be to purchase pre-made molecule models. Our collection, which allows us to build all of the 20 essential amino acids simultaneously, includes one organic chemistry student set, a glucose model and a fat molecule model from Carolina Biological Supply, and a few additional atoms from Indigo Instruments. (This option was actually less expensive their “20 amino acid” kit!)
When purchasing Molymod kits be sure to investigate a wide variety of vendors. We found that prices for identical items varied widely, and no one vendor offered the best price for every kit, especially when shipping charges are considered.
We’ve seen dozens of student molecular model sets online that look a lot like Molymod (including from Ward’s Science), often for considerably less money. Are they made by Molymod? Are the components interchangeable? Are they of comparable quality? We do not know. There are too many to try them all, but we have ordered some kits to test and will update this post with our findings.
| FHT | Molymod | Ward’s | Happy Atoms | |
|---|---|---|---|---|
| Hydrogen | Orange | White | White | White |
| Carbon | Black | Black | Black | Black |
| Nitrogen | Dark Blue | Blue | Orange | Blue |
| Oxygen | Light Blue | Red | Red | Orange |
Does your experience with these models match ours? Are there other kits you think are also worth looking at? We’d love to hear from you! Drop us a note or leave a comment!















0 Comments